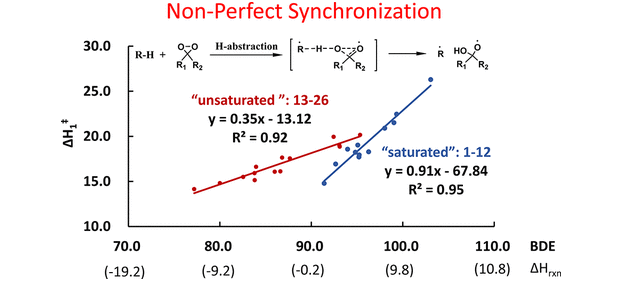
The selectivities in C–H oxidations of a variety of compounds by DMDO have been explored with density functional theory. There is a linear Evans–Polanyi-type correlation for saturated substrates. Activation energies correlate with reaction energies or, equivalently, BDEs (ΔH‡sat = 0.91*BDE – 67.8). Unsaturated compounds, such as alkenes, aromatics, and carbonyls, exhibit a different correlation for allylic and benzylic C–H bonds (ΔH‡unsat = 0.35*BDE – 13.1). Bernasconi’s Principle of Non-Perfect Synchronization (NPS) is found to operate here. The origins of this phenomenon were analyzed by a Distortion/Interaction model. Computations indicate early transition states for H-abstractions from allylic and benzylic C–H bonds, but later transition states for the saturated. The reactivities are mainly modulated by the distortion energy and the degree of dissociation of the C–H bond. While the increase in barrier with higher BDE is not unexpected from the Evans−Polanyi relationship, two separate correlations, one for saturated compounds, and one for unsaturated leading to delocalized radicals, were unexpected.